98% of myo/pericarditis foreign VAERS reports were published after the PVP analysis
258 reports were received between Dec 2020 and May 2021. 254 of them were only published after 21st June 2021, which was the date of the Pharmacovigilance Plan (PVP) analysis.
Summary:
There were long delays between the date the report was received and the date it was actually published for a lot of foreign VAERS reports in early 2021
The usual explanation - that the system got overwhelmed because of too many reports - already reflects very poorly on the CDC because they had many months to anticipate and prepare
Even if you allow for this explanation, the delay pattern that you see for Myocarditis and Pericarditis reports looks very strange. This is quite clear when you compare it with the delay pattern for Death reports
Does the delay pattern indicate an intent to deceive?
In my previous article I pointed out that there was a 23X undercount of myocarditis and pericarditis foreign VAERS reports in the Pharmaco-vigilance Plan (PVP) analysis done by Pfizer and submitted to the FDA.
Pfizer's mid-2021 PVP report to FDA under-counted foreign VAERS cardiac reports by 23X
Summary: Pfizer’s submitted a revised pharmaco-vigilance plan (PVP) report to the FDA in mid-2021 which analyzed myocarditis and pericarditis reports until ~mid June 2021 It included an analysis of foreign VAERS, but ignored a vast majority of reports which had been filed by that date
Here are the raw numbers (please refer to the previous article to see the corresponding screenshots)
Select the months Dec 2020 - May 2021 in the RECVDATE field.
Now select Myocarditis and Pericarditis in the SYMPTOMS field.
Then choose Pfizer in the vaccine manufacturer field.
There are 258 such reports.
The PVP analysis was done on 21st June 2021. Only 4 of the reports had been published by that date. The other 254 were published later.
Now let us look at the published date for these reports.
How to find the published date for a given VAERS report
In my view, the easiest/simplest way to find the published date for a given VAERS report is to go to the MedAlerts VAERS Wayback Machine for the given VAERS ID.
Here is an example URL:
https://medalerts.org/vaersdb/findfield.php?IDNUMBER=2052072&WAYBACKHISTORY=ON
To find the published date for any VAERS report, simply replace the VAERS_ID in the URL with the ID that you are interested in.
Note: I got a list of published dates from the OpenVAERS team, and it may not exactly match the one on the MedAlerts website. But the date is only off by a day or two.The published date is what determines whether a given VAERS report will appear in the CDC WONDER VAERS search. But the CDC WONDER VAERS search tool does not even provide an option to filter by the published date when people use its tool!
Once you read this article, you will realize the importance of the report published date.
Published Date vs RECVDATE
This would be a good time to remind people that the published date is what matters for Pfizer’s PVP analysis.
In other words, even after the report is received - which is represented by the RECVDATE in the CSV file, sometimes the report does not appear in the CDC WONDER VAERS search for whatever reasons.
In turn, Pfizer can claim that the analysis they did was accurate on that date, and that claim is true.
But we do know that there were a lot of delays in publishing these reports because apparently no one expected such a high volume of reports to be sent in.
What caused the delays?
As always, our friend David Gorski jumps to the defense of the CDC in his “rebuttal” of the recent BMJ article on VAERS
I doubt that anyone would deny that, early after the rollout of COVID-19 vaccines, the system was overwhelmed. After all, this was the largest and fasted rollout of a vaccine since VAERS was created over three decades ago. It was also the first time that the government had so heavily advertised VAERS with each dose of COVID-19 vaccine and encouraged people to report any adverse events (AEs) that they thought to be due to the vaccine. The CDC even created another system (V-Safe) that, unlike VAERS, a passive reporting system, send periodic text messages to vaccinees who signed up to ask them how they were doing. This provided more cues to report to VAERS than had ever been provided before. Now, don’t get me wrong. If the system was overwhelmed and understaffed to the point where its servers would time out and its staff couldn’t follow up in a timely fashion on AE reports, then that should have been fixed.
Well, isn’t that interesting?
Because we are also told that the reason for the huge volume of COVID19 VAERS reports was simply because of the number of doses administered, and that this would be obvious to anyone with half a brain if only they looked at what happened with VAERS reports during the 2009 swine flu epidemic.
So let us put it all together:
the CDC had already published an article before COVID which was used as an explanation for prior sudden jumps in VAERS reports using the 2009 swine flu as an example
they already had an estimate of the number of VAERS reports they could handle with current staff
“Before the pandemic VAERS was receiving nearly 60 000 adverse event reports each year. A 2015 CDC article suggests that the agency had the capacity to request records for just a few thousand serious reports each year. But in 2021 the total number of reports shot up to a million, and another 660 000 have been filed since. Nearly one in five meet the criteria of serious. This surge reflects the unprecedented campaign to vaccinate against covid-19—in the US alone some 675 million doses have been administered—and the vast majority of recent reports are related to covid vaccines. The CDC states that, “in the event of a significant increase” in VAERS reports warranting clinical review, the standard operating procedure requires additional CDC Immunization Safety Office staff to process cases.“
apparently, the health regulators advertised VAERS even more heavily this time around
there was a two Trillion (with a T) dollar pandemic spending bill in 2020
But:
No one from the CDC thought it would be a good idea to use some of that money to hire contractors and train them on VAERS before the vaccine rollout so that the system did not get overwhelmed1?
Very interesting.
But even if you allow for this delay, there is still something strange about myocarditis and pericarditis reports.
Why myocarditis and pericarditis?
If you are quite familiar with VAERS, and spend some time reading the entire PVP report, two things immediately stand out:
the report uses a lot of imprecise language to describe the VAERS queries which were run. For example, we often cannot ascertain whether the query was run on both the US and foreign datasets, or whether it was US only
the “query date” was not the same for all the conditions which were analyzed (anaphylaxis, myo/pericarditis, pregnancy, under-12 cohort, vaccine effectiveness), even though that would have been much more logical given that the entire document was eventually submitted as a single PDF file
So you can sense that the final report is quite disjointed, as if multiple teams of people had just “slapped in” their respective sections without reading the rest of the document to see if the full report was actually readable.
On top of all this, the myocarditis and pericarditis section is even more disjointed, given that it is the only section in the entire document which cites individual VAERS reports.
My immediate question was - why did myocarditis and pericarditis get this special consideration? (obviously they are dangerous adverse reactions, but there are also other dangerous adverse reactions)
I got two responses on Twitter which made a lot of sense.
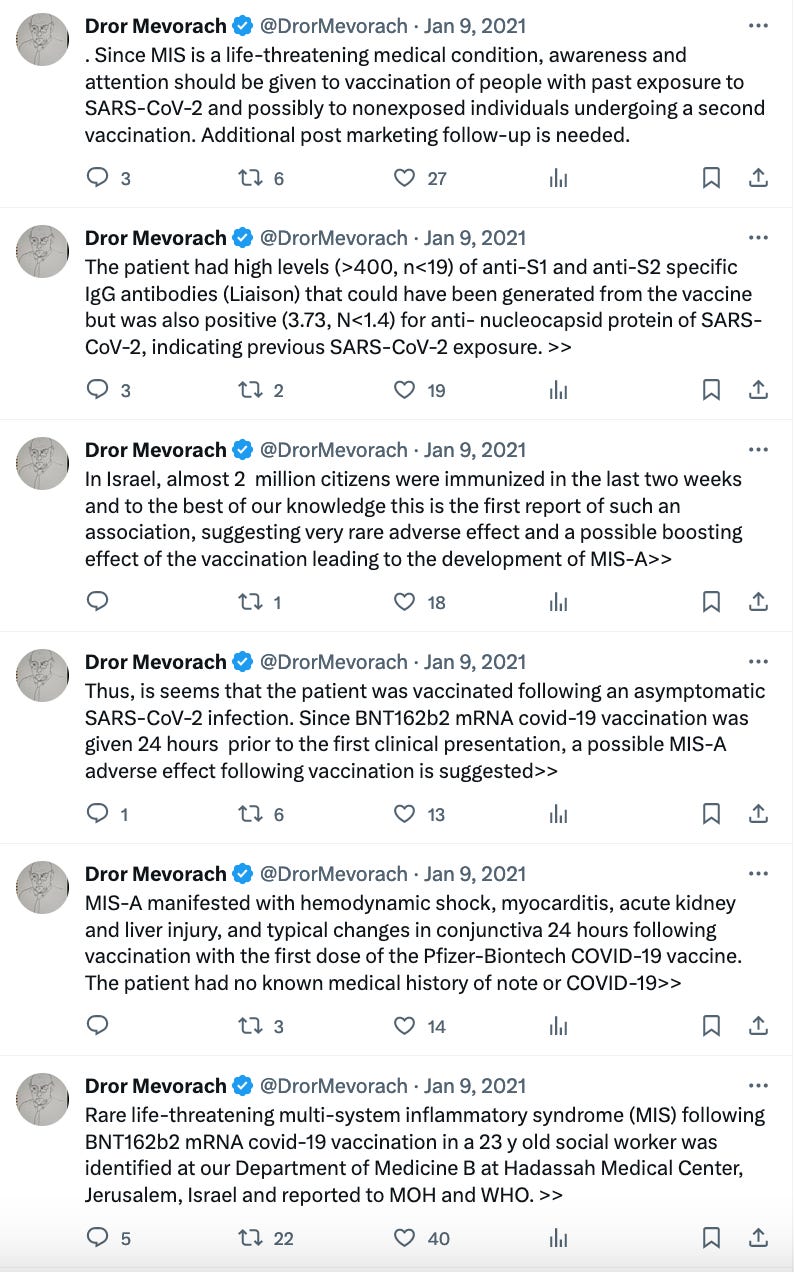
Apparently a Doctor in Israel had first raised the issue in Jan 2021 itself:
For some reason, the Doctor had the urge2 to post a series of Tweets about this as early as 9th January 2021. He did not post it as a single thread, so it appears in reverse chronological order below.
It is also quite notable that four months from 9th Jan 2021 is around mid-May 2021, and the submission date of the original PVP report was 18th May 2021.
It looks like the FDA asked Pfizer to submit a revised PVP report which included myocarditis and pericarditis because someone outside the US had already analyzed the data and statistically proven the link to myocarditis.
At this stage, ignoring the problem would have raised a lot of doubts about whether the FDA was really fit for its (then) unofficial title as the world’s premier drug approval authority3.
Was there an intent to deceive?
Recently Kevin McKernan published an article on his Substack titled “Intent to Deceive”
If you read the article, you can see that some actions by Pfizer are very hard to explain as being due to coincidence or random chance.
In the same way, I see some strange patterns with the myocarditis and pericarditis reports.
Consider all death reports in foreign VAERS from Dec 2020 - May 2021.
There are a total of 3815 for the Pfizer vaccine.
Now consider the date ranges when these reports were published in VAERS after which they became searchable.
Let us refer to these tranches as follows:
Tranche 1 - publish date before 18th May 2021
Tranche 2 - publish date between 18th May 2021 and 21st June 2021
Tranche 3 - publish date after 21st June 2021
So the ratio of reports in the three tranches are as follows:
Before 18th May 2021 (Tranche 1): 133/3815 = 3.5%
18 May - 21 June 2021 (Tranche 2): 1143/3815 = 30%
After 21 June 2021 (Tranche 3): 2539/3815 = 66.5%Notice that Tranche 2 has nearly 30% of the death reports.
So even if you admit that the delays in publishing were just completely random delays because “the VAERS system got overwhelmed”, you can see that the most severe outcome4 (death) still had nearly 30% of the delayed reports in Tranche 2.
Now compare this with myocarditis and pericarditis:
There are a total of 254 reports for Pfizer (some reports have both symptoms).
Look at the tranche distribution:
Here are the numbers:
Tranche 1: 0%
Tranche 2: 1.5%
Tranche 3: 98.5%So not only did they5 make sure none of the Myocarditis and Pericarditis reports were published before the first PVP report (which was submitted on 18th May 2021), which is already problematic given 65 of them had already been filed by end of March.
But they only had a handful of reports in Tranche 2 so that they could list all of them in the revised PVP report and create the false impression of painstakingly complete pharmacovigilance.
Are we supposed to believe this is all mere coincidence?
Also remember - this revised PVP report, which revealed these suspicious delays in publishing VAERS reports, was itself delayed until the last possible stage of the FOIA response, and was only published in November 2023!
Plus do other things - like provision more server capacity, put some temporary alternative system in place if the servers still got overwhelmed, communicate all these concerns to the relevant authorities etc. The best time to do these things is before the systems get overwhelmed, and they had a good 9 months to prepare.
Despite being the first to identify the link to myocarditis, the same Doctor eventually also supported the Pfizer vaccine for other age groups, but urged caution for young people. Some people think everyone who agrees with vaccination must be some kind of “controlled opposition”, but it is quite clear that for a lot of people this was not a binary Yes/No decision, especially considering the context of the global panic.
It would be a good time to remind the readers that in mid-2021 a lot of people (including me) did consider the FDA as something of an authority who knew better than all the other health agencies in the whole world. For example, this June 2021 YouTube video by an Indian TV channel was quite deferential to FDA’s opinion and was not yet doubting if the FDA was unilaterally picking market winners for the vaccine rollout.
One possible explanation for these delays, is that the time was spent gathering information about patient history and underlying conditions so as to do a complete assessment and to submit the complete information to VAERS. But in that case, you would expect the delays for Death reports to be more, not less, than myocarditis and pericarditis given that there is no outcome which is more severe than death.
Who is “they”? To be honest, I don’t really know.
But here is what the CDC says about foreign VAERS reports. “VAERS occasionally receives case reports from US manufacturers that were reported to their foreign subsidiaries. Under FDA regulations, if a manufacturer is notified of a foreign case report that describes an event that is both serious and unexpected (in other words, it does not appear in the product labeling), they are required to submit it to VAERS. It is important to realize that these case reports are of variable data quality and completeness, due to the many differences in country reporting practices and surveillance system quality.“
Looks like “they” are Pfizer’s foreign subsidiaries if I am interpreting that correctly.

















VAERS hired an outside contractor called General Dynamics to handle expected surge in reports. Ideally, someone from GD could help explain what appears to be a deliberate throttling of reports around the date of Pfizer's myocarditis report in June 2021. Presumably the inclusion of 254 more reports would shift the level of concern greatly.
Pfizer employed 2,400 extra staff just to handle the millions of Adverse Events flooding in.