Recently, someone asked me this in the comments section of my article about UK VAERS death reports
Hi Aravind, I agree with your conclusion.
Are you also aware that for the first part of 2021 the majority of UK covid vaccinations (and certainly the majority of deaths reported) were using the astra zeneca formula? I assume the AZ adverse effects were not reported to Vaers?
I started looking into this and went down quite a rabbit hole!
Algorithm
I am going to provide a very high level description of the algorithm I used because the code turned out to require (handling) quite a lot of corner cases and I do not want to spend all the time describing the code.
First, I took the foreign VAERS reports and filtered it into a DataFrame which has the word AstraZeneca (and other common names used for it) in the SYMPTOM_TEXT field.
df = df.loc[(df['SYMPTOM_TEXT']).str.contains('astrazeneca', na=False) |
(df['SYMPTOM_TEXT']).str.contains('vaxzevria', na=False) |
(df['SYMPTOM_TEXT']).str.contains('ncov19', na=False) |
(df['SYMPTOM_TEXT']).str.contains('ncov-19', na=False) |
(df['SYMPTOM_TEXT']).str.contains('chadox1', na=False)]Then I iterated through this DataFrame and looked for the phrases similar to “Dose 1”, “1st dose”, “Dose 2”, “2nd Dose” etc.
My goal here was to extract the vaccine name and the vaccination date for each COVID19 vaccine dose.
Results
Here are the results from my analysis. There were a total of 7312 foreign VAERS reports which had the word AstraZeneca (or its variation) in them.
I allow up to Dose 4 in my analysis. There are already very few reports with Dose 4. If there are people who took more doses, they don’t show up in the analysis.
AZ_REPORT
The question which prompted this entire analysis is whether AstraZeneca reports are found in VAERS.
So I did something really simple: if the word AstraZeneca is present in the SYMPTOM_TEXT but of all the vaccine names I identified, none of them is AstraZeneca, then that is a report specifically for the AZ vaccine.
There were exactly 2 reports.
Report 1
Patient called the day after astrazeneca dose 1 injection and reported adverse effects of sore arm at the site of injection and tireness. Pharmacist counselled that these are common side effects. if adverse effects worsen or progress, to contact the pharmacy again. Patient called again on April 15, 2021 and informed pharmacist of additional side effects that started 4 days after the injection. Pins/Needles in arms/legs/feet, which has now then progressed to whole body. Recurring episodes, she is uncomfortable and the pins/needle sensation is making her difficult to have restful sleep.
Report 2
Off label use; Interchange of vaccine products; Seronegative arthritis; This is a spontaneous report received from a contactable reporter(s) (Other HCP) from Regulatory Authority. Regulatory number: 708910. A 76 year-old male patient received COVID-19 Vaccine - Manufacturer Unknown, (Batch/Lot number: unknown) as dose 2, single for covid-19 immunisation. The patient''s relevant medical history and concomitant medications were not reported. Vaccination history included: Covid-19 vaccine astrazeneca (Dose 1), for COVID-19 immunisation. The following information was reported: OFF LABEL USE (medically significant), outcome "unknown", described as "Off label use"; INTERCHANGE OF VACCINE PRODUCTS (medically significant), outcome "unknown", described as "Interchange of vaccine products"; SERONEGATIVE ARTHRITIS (medically significant) with onset 01May2021, outcome "not recovered", described as "Seronegative arthritis". No follow-up attempts are possible; information about lot/batch number cannot be obtained. No further information is expected.
In my opinions, these exceptions only prove the rule. So I am going to say there were no AstraZeneca reports in VAERS.
But I did point this out to the commenter:
That is correct.
VAERS does not have reports for the AstraZeneca vaccine, and I don't know why.
I saw a few weird VAERS reports where the complaint is about the AZ vaccine (because the narrative text says so), but they chose the Pfizer as the name of the vaccine in the VAX_NAME column. This could be because it is some kind of dropdown field where AZ does not even pre-populate.
If anyone reading this knows more about this topic, please leave a comment.
In other words, what if there are reports where the person reported a different vaccine name because they did not have an option to choose AstraZeneca in the form they filled out?
I will answer this question later.
UNKNOWN_VAX
If my Python script is not able to identify the vaccine name for any of the doses, the field UNKNOWN_VAX is marked as True.
There are only 239 such reports.
MISSING_DOSES
COVID19 vaccines were already known to be at least a 2-dose schedule before the vaccine rollout began. But there are a LOT of reports where the full sequence of COVID19 vaccinations is not reported in VAERS. I have used a field called MISSING_DOSES to mark these entries for the filtered dataset.
This is how I calculate the value: I check the VAX CSV files (which lists all the vaccinations for a given VAERS_ID) to see how many COVID19 related vaccinations that person got. Then I check the number of vaccine doses calculated by my Python script.
If the Python script identifies more doses than what I see in the VAX CSV file, I mark MISSING_DOSES as true.
While this algorithm cannot have false positives - reports marked as True even if there are no missing doses - it can have false negatives (i.e. reports marked as False even if there are missing reports).
df_curr_id: pd.DataFrame = df_covidvax.loc[df_covidvax['VAERS_ID'] == vaers_id]
num_doses_recorded = len(df_curr_id)
missing = True if num_doses_recorded < num_doses else FalseThere are 6901 reports which are missing the full vaccination dosing schedule in VAERS, which is nearly 95% of the AstraZeneca reports.
While I don’t know if this is representative, it is a very large percentage if this holds true for the entire foreign dataset.
Remember that when the MISSING_DOSES is marked as True, that usually implies a report for Dose 2 or later.
But if all information about the first dose is missing from the VAERS VAX file, this means a lot of data analysis of VAERS (e.g. people who do data mining on the VAERS dataset for COVID19 vaccines) are probably working with incomplete information unless they also do some text analysis similar to mine.
INCOMPLETE
The INCOMPLETE field is marked true when I could clearly identify the mention of dose number (e.g. “Dose 3”) in the SYMPTOM_TEXT, but I was not able to get the vaccine name for that dose.
There are only 556 such reports, but note that within a single report there could be multiple doses for which I wasn’t able to identify the vaccine name.
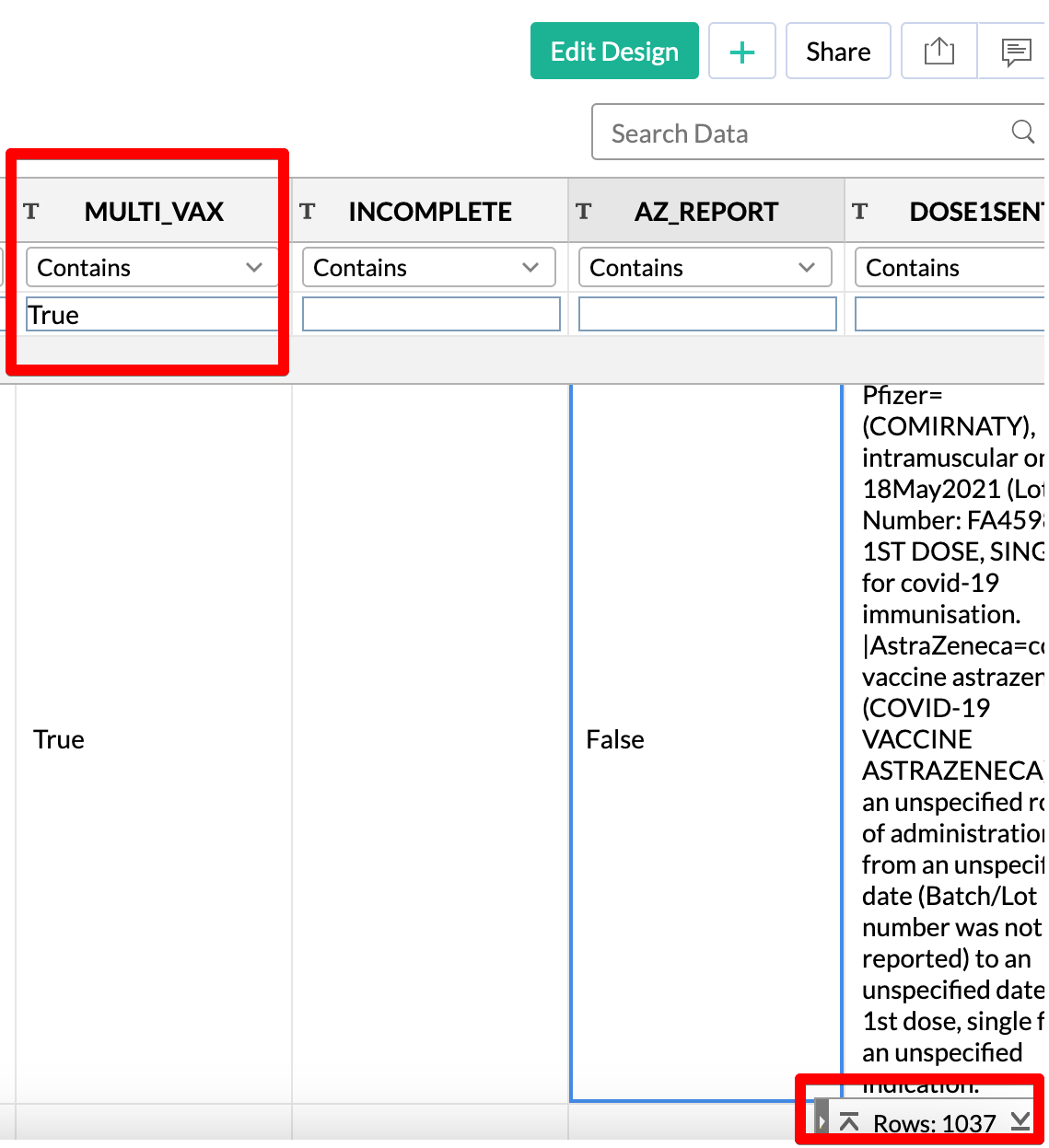
MULTI_VAX
In the AZ_REPORT paragraph, I mentioned that there were actually a few “weird” looking reports where the same dose number corresponded to two different vaccines according to the SYMPTOM_TEXT.
I created a list of these “collisions” under the MULTI_VAX field
You can check out the DOSE1VAX (or other dose name fields) to see which vaccines are colliding.
For example, take a look at the screenshot below. There are three reports highlighted where the first dose vaccine was identified as both AstraZeneca and Pfizer in the SYMPTOM_TEXT.
If you look at the writeup for one of them
shortness of breath; Malaise; throat constriction; flushing; Revaccination with different COVID-19 vaccine; This is a spontaneous report from a contactable physician downloaded from the Agency Regulatory Authority-WEB FI-FIMEA-20212979. A 57-year-old female patient received the first dose of BNT162B2 (COMIRNATY), via an unspecified route of administration on 28May2021 as single dose for COVID-19 immunisation; received the first dose of covid-19 vaccine nrvv ad (chadox1 ncov-19) (VAXZEVRIA, AstraZeneca) via an unspecified route of administration on 04Mar2021 as single dose for COVID-19 immunisation. Medical history included fibromyalgia, sleep apnea, DM2, hypercholesterolemia, overweight, tape allergy. No drug allergies. Concomitant medications were not provided. The patient had revaccination with different COVID-19 vaccine. The patient received COMIRNATY vaccine on 28May2021. On unspecified date, patient experienced 20 min throat constriction, shortness of breath, flushing. Events shortness of breath and malaise with hospitalized. Medicated with Epipen, Cetirizin and Solu-Cortef iv. There was no need for new Epipen or iv cortisone. Unclear malaise, which was why patient was monitored overnight in the hospital. Short-term tablet cortisone and antihistamine treatment continued. Treatment received for events shortness of breath, malaise, throat constriction, flushing. The outcome of events shortness of breath and malaise was recovering, while of the other events was unknown. No follow-up attempts are possible; information about lot/batch number cannot be obtained.
Notice the places I have highlighted. You can see that both vaccines are mentioned as being the first dose. But there are two different dates which are separated by a good few weeks.
So my current assessment is that these were just mis-reported as being first dose. There are quite a lot of reports which follow this same pattern where they have two different dates mentioned for the different doses.
This suggests two things straight away:
a) the 2nd dose was wrongly reported as being the 1st dose quite often.
In turn this means the adverse event corresponding to Dose 2 was wrongly attributed as an adverse event corresponding to Dose 1.
In turn, this probably means the 2nd dose is even more dangerous than what people believed before.
Of course, this also depends on how often this misreporting happens. I will do a more thorough analysis of this in a future article.
b) the most recent dose was Pfizer and the VAERS report is for the Pfizer vaccine
As always, comments and feedback welcome.







Also 250 AZ lot codes with expiration dates can be helpful in ID'ing them.
https://thingsweirdandwonderful.com/lot_codes/
As I understand it, it's correct that Astrazeneca doesn't appear as an option in the dropdown. There's an option to download a writable PDF at CDC where one could take a look. Appears sometimes people choose one of the other companies
If you used the Nov 11 dataset you might get a different result?