A critique of the CDC paper on bivalent booster for 5-11 year olds
This paper could not have cleared a honest peer review system
A couple of months back, CDC’s MMWR released a paper which analyzes VAERS reports for the 5-11 cohort. It was cited in the letter that the FDA and CDC recently sent to Dr Joseph Ladapo.
The letter cites the CDC’s website:
And this is the paper we will be looking at:
I have a lot of criticisms of that paper, and I will list them here one by one.
But before I do, I want to remind folks about the article I recently wrote pointing out that nearly all papers written about VAERS understate vaccine dangers.
This is another such paper (it also cites the v-safe, which I will also address in this article).
Before we go further, please note that this is the Limitations section from the paper:
The findings in this report are subject to at least four limitations. First, v-safe is a voluntary program, and data might not be representative of the vaccinated population. Second, v-safe does not directly identify whether a vaccine is monovalent or bivalent; therefore, misclassification might occur among children who aged into this population without having completed a 3-dose primary series. Third, VAERS is a passive surveillance system and subject to reporting biases and underreporting, especially of nonserious events (5). Finally, conclusions drawn from these data are limited by the 11-week surveillance period; safety monitoring will continue during the bivalent booster vaccination program.
Here is the list of issues I found in the paper:
1 The number of VAERS reports is higher
From the paper:
During October 12–January 1, 2023, VAERS received and processed 922 reports of adverse events among children aged 5–11 years (Table 3).††† The median recipient age was 9 years (range = 5–11 years), and 459 (49.8%) reports were for females. Approximately 13.4% (124) of registrants received at least one other vaccination during that same visit; of those, 115 (92.7%) received an influenza vaccine. Among all 922 VAERS reports, 920 (99.8%) were classified as nonserious, 845 (99.8%) after Pfizer-BioNTech and 75 (100%) after Moderna bivalent booster vaccination.
But the bivalent VAERS reports were being received even before 12th October 2022. Why start on a later date?
The VAX_TYPE for the bivalent booster VAERS reports are all marked as COVID19-2. So I will use that in my query, and also narrow it down to the age range and date range mentioned above. (Age between 5 and 11, RECVDATE before ‘1-Jan-2023’)
There are actually 1021 such reports if you consider all the reports received until 1st Jan 2023, which is almost 100 reports more than what the MMWR paper says. Why not include all of them?
So the total number of reports they have used is already wrong. The paper fails to mention this in their Limitations.
And where do these 100 extra reports come from?
Suppose I look for reports filed before and until 11-Oct-2022, I still see only 62 of them.
This means another 35+ reports were added later into VAERS. This is because there is a lag of 4-6 weeks before a report is received to VAERS and they eventually publish it.
The Limitations section also fails to mention that there is a backlog in VAERS which causes some reports to enter the system a few weeks after they are first reported.
I have published all the 1021 reports below.
2 The AGE_YRS field is not always translated
There are 386 reports for the bivalent vaccine where the AGE_YRS field is missing and left as null.
Obviously, just because these are missing does not mean they are in the age range which is being discussed. But the Limitations section does not mention that the AGE_YRS is not always translated, which can sometimes lead to an undercount.
As a quick aside, the AGE_YRS field is missing for a really large number of foreign reports, which means anyone doing research on foreign VAERS might be missing out on a lot of information. At least this issue is not common in US VAERS reports.
Also, sometimes the age is not translated into the AGE_YRS field even though it is mentioned in the writeup. It is pretty rare in the reports for the cohort being considered.
3 Followup VAERS reports are ignored
I have written about this extensively in my recent articles. If someone files a followup report to an original VAERS report, only the original report is published.
This policy is questionable, but once again, my problem is that it is not even mentioned in the Limitations section of the paper.
This matters, because sometimes the patient condition deteriorates.
I have not seen any followup reports for the bivalent booster for the 5-11 cohort yet, but sometimes followup reports are filed many months later. Just because there aren’t any reports now does not mean there will not be some in the future.
Here is an example.
This is the original report and this is the followup report.
Vaccination date: 2021-02-26
Symptom onset: 2021-02-26 (same day, patient goes into coma)
First report on: 2021-02-28 (2 days after symptom onset)
Followup report on: 2023-01-09 (after patient died in Oct 2022. Looks like she might have been bedridden the whole time and never walked again)
The lag time between original and followup reports is nearly 2 years, but if we did not have the followup report, we will not have the correct picture of the outcome.
4 There are probably more serious adverse events than reported
Here is another paragraph from the paper (emphasis mine)
After administration of >950,000 doses of bivalent booster vaccine to children aged 5-11 years, only two serious VAERS reports have been received. Approximately 99.8% of reports to VAERS for children aged 5–11 years after bivalent booster vaccination were deemed nonserious; most (85.0%) reports were related to vaccination errors. Many vaccination errors represented children receiving an incorrect bivalent booster dose for their age or an incorrectly reconstituted dose. Most reports of vaccination error did not include an adverse health event; those with an event were consistent with expected reactions after an mRNA COVID-19 vaccination. Among events reported to VAERS, vaccination errors were reported with a similar frequency among children aged 5–11 years after monovalent (71%) or bivalent (84%) booster vaccination (7). Vaccination errors represented a smaller proportion of events (35%) reported among persons aged ≥12 years who received bivalent booster vaccination (4). CDC provides updated clinical guidance, educational materials, and training opportunities after each update to COVID-19 vaccine recommendations.¶¶¶ Public health officials should continue to provide training materials for vaccine administrators to help reduce vaccination errors among children.
The claim is that only two serious adverse event reports have been received.
This is technically true. The only adverse events which qualify as serious are those where one of the following fields is marked as “Yes” - died, hospitalized, disabled or life-threating. There were three such reports in total, but it is quite possible one of them had not been received by the time the paper was written.
However, there are at least 19 cases where the kid fainted, often on the same day of vaccination. These have the symptom name “Syncope”. And there are another 12 cases where the kid fell (also due to fainting), and these have the symptom name “Fall”.
Are these fainting episodes serious? We will not know until they do a followup.
But followup reports are not being added to VAERS. There are at least a handful of writeups which are
a) reported immediately after the incident
b) suggest there will be a followup report
Pt received booster yest. Pt had poor appetite at breakfast this am, took ibu d/t sore arm. nausea at lunch today, then passed out, no injury r/t fall. pt picked up from school, will sched appt for tomorrow.
The Pfizer bivalent 10 mcg/0.2 ml (properly diluted and mixed by the pharmacist), was given intramuscularly to patient. Patient''s guardians was appropriately counseled after the vaccine and left the vaccine room with no questions. Approximately 15 minutes later, patient fainted and hit her head and vomited outside the pharmacy vaccination room. Immediately, pharmacy staff went over to assist the patient and guided her to sit down in the immunization room. Patient and guardian sat in the immunization room for ~30 minutes and nausea resolved. Patient and family left with no questions. Pharmacist will follow up with patient''s guardians the following day after the vaccination. VAERS report was filed.
Patient received vaccination and 5 to 10 min after fainted. EMS called. Vitals: BP: 92/48, HR fluctuating between 77-105, O2 - 96,
Once again, the paper should have at least mentioned in the Limitations section that not all the followups had been completed.
5 The followup reports cannot be consistently matched to the originals
Even though both original and followup reports contain important information, the CDC chooses to delete followup reports. They should ideally figure out some way to merge the reports, but they don’t do that.
Some people have calculated the difference (missing VAERS IDs) between two weekly reports and have constructed the full dataset of deleted reports, so technically it is possible to match followup reports to original reports.
Someone might read this and ask “If the followup reports are still available, can we not just go and see the followup report and verify if everything is OK?”
Unfortunately CDC does not publish any mapping between the followup and original report either, so the only way to do a full followup is to use the (sometimes incomplete) fields in these reports and “guess” the matches. This process is able to identify the duplicates for only about 50% of the deleted followups.
The paper should have also mentioned this in the Limitations section.
Of course it would become very tricky for the CDC to admit what is happening with these followup reports. People in the VAERS research community know the CDC collects additional never-published information, such as phone numbers, which can probably be used to identify followups. Since this additional information is not published, the external researchers do not quite have the same capabilities when it comes to matching the followup to the original report. But if they mention all this in the Limitations, they would likely be forced to disclose their deduping algorithm.
6 Even the benign adverse events could hide more problematic ones in the future
This policy of deleting followup reports becomes even more worrying once you notice that a lot of VAERS reports were for kids who got injected with the adult dose.
In fact, the paper points this out:
Approximately 99.8% of reports to VAERS for children aged 5–11 years after bivalent booster vaccination were deemed nonserious; most (85.0%) reports were related to vaccination errors.
Even if a small fraction of this 85% of reports do end up causing more severe adverse reactions, those reports will not show up in VAERS because of the way it is currently dealing with followup reports.
What Dr Jessica Rose is pointing out here is true.

But considering that these are all little children, it is actually worse in a way.
By not even acknowledging that followup VAERS reports are almost never added into the system, the CDC is now also making sure that no external researcher ever studies these adverse reactions in complete detail!
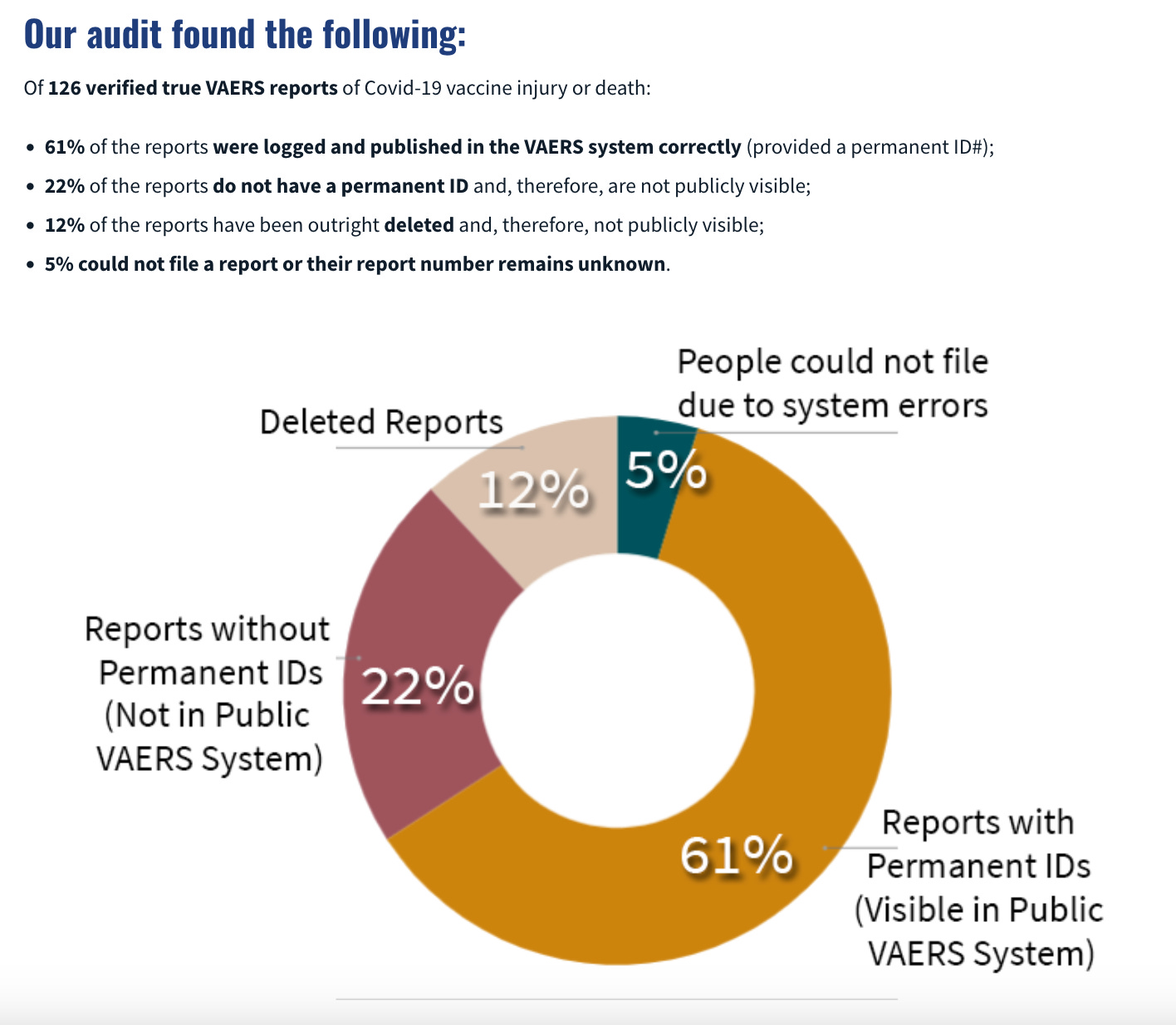
7 Not all VAERS reports even make it into the public dataset
Recently, the folks at React19.org did an independent VAERS audit by asking people they knew (who had suffered vaccine adverse reactions) to file a VAERS report.
Here is what they found:
First of all, it should be concerning that there are so many people who had adverse reactions that this audit was even possible.
But you can also see that some reports did not even get added into the public VAERS dataset.
Once again, if there is a good reason for this, it must have been mentioned in the Limitation section.
Maybe at this point you are thinking: “well, the CDC does have multiple monitoring systems and VAERS is passive surveillance anyway. There are also other checks and balances in the system to keep them honest.”
Well, let us see if you still hold that opinion by the time you get to the end of this article.
So let us look at the section where the paper discussed V-SAFE.
This is how the paper describes V-SAFE
To characterize the safety of bivalent mRNA booster doses among children aged 5–11 years after receipt of bivalent Pfizer-BioNTech and Moderna booster doses, CDC reviewed adverse events and health impacts reported to v-safe,* a voluntary, smartphone-based U.S. safety surveillance system established by CDC to monitor adverse events after COVID-19 vaccination, and to the Vaccine Adverse Event Reporting System (VAERS), a U.S. passive vaccine safety surveillance system co-managed by CDC and FDA†
Unfortunately, the v-safe data is not public in the same way that VAERS is.
Despite all the limitations I have mentioned, VAERS is the closest we have to an open source vaccine injury database.
So the rest of this article is a critique of the v-safe process and not specifically of the information they provide in the paper (which cannot be independently verified in any case).
8 CDC did not even add all the possible severe adverse reactions in the V-SAFE check-the-box options
Even though Pfizer themselves mention a million potential adverse reactions to look out for, of which a mere 750+ failed to clear the not-exactly-super-high PRR bar, the V-SAFE app for COVID19 vaccines has only a list of a few possible symptoms and even those were just normal after-vaccine reactions.
Bottom line, and as you will already know from the prior parts, the only check-the-box data that could even potentially raise a safety concern would be a high rate of individuals reporting that they sought medical care after the shot.
This is because the 10 check-the-box symptoms, gathered for seven days after injection, are the same symptoms the CDC say are a good sign after injection because they show the body is developing immunity. That is why the CDC explains that “Any side effects from getting the vaccine are normal signs the body is building protection.” And indeed, when very high rates of these symptoms were reported to v-safe, CDC said there is no cause for concern.
Actually the story is a little worse, but this part is also a bit speculative.
It looks like CDC originally designed the v-safe app to allow people to select more adverse reactions, but finally released the version which did not have the full list.
9 CDC did not make the v-safe data public
Unlike VAERS, CDC does not make the v-safe data public. So you do have to take their analysis at their word.
But that is still not too bad. At least in the US, you can still get this information by sending FOIA (Freedom-of-information-act) requests to the CDC.
10 CDC delayed sending the FOIA data
So some folks who have been looking at vaccine injuries for many years now decided they will just send a FOIA request to the CDC to get access to this FOIA data.
But the CDC delayed releasing this information by more than a full year.
So, it all begins with a simple request to our federal health agency made a long time ago. On behalf of ICAN, in June 2021, we sent the following request to CDC asking for all de-identified data submitted to v-safe since January 1, 2020:
After a lot of back and forth, this is what happened (same article):
And as agreed and ordered by the Court, on September 30, 2022, I received a letter which provided in relevant part as follows:
In other words CDC waited until this happened:
And then until this happened:
And only then they sent the information requested.
11 Even the data the CDC eventually sent was still incomplete
Now this might have been OK if the FOIA data that the CDC sent was actually complete.
Remember how they used a very small list of check-the-box options to record adverse reactions?
They did have a free text field where people could type in anything they wanted. And some people did use them to report the more serious stuff.
Except, the CDC did not send information from the free text field over to the lawyers who made the FOIA request (emphasis mine)
Had v-safe not existed, there is a high likelihood that more people would have reported to VAERS and, again, theoretically, the public would have access to that data. We may never know the content or precise number of free-text field submissions in v-safe, which are still hidden from public view (although we continue to fight to obtain this data), whereas we would have access to public-facing VAERS reports.
As you can see, the more free text information entered into the v-safe blackhole, the less information entered into the public facing VAERS dataset.
Oh, and in the meantime, they did two more things.
The CDC stopped publishing the narrative text writeup for foreign VAERS reports:
And they recently also added the mRNA vaccines into the childhood immunization schedule.
Summary
Since I don’t have a background in medicine or biology, all this information alone is not sufficient for me personally to make any decision about all these vaccine recommendations.
I am looking at everything from the viewpoint of text analysis (which is my specialization), but also I saw an interesting comment recently on Alexandros Marinos’ Substack.
People might know that Alexandros has spent a lot of time looking into papers written about Ivermectin. And even after reading all that analysis, I am still not sure what to make of the efficacy of Ivermectin for COVID19.
But if I use “incentive driven analysis” as a tie-breaker, and look at how much the drug was maligned in the press, I will say
“Yes, there is a very high probability that the papers which are pro-Ivermectin produced genuine results even if the trials were a bit sloppy, and the papers which are anti-Ivermectin produced biased results even if the trials seemed much more professional”
And I hold a similar opinion on vaccine injuries. I think there are way too many misaligned incentives preventing the US health regulators from doing their jobs, and I do not consider their analyses to be objective.
Why is no one in the pro-vaccine community concerned about all this?
I understand that it can be hard to switch sides after taking a strong stance, but does this really look like a corruption-free system to you?













Friendly reminder from FDA to tell the doctor holding the needle if your 5 year old is pregnant
https://www.fda.gov/media/153717/download https://archive.ph/rxD3j